Diabetes Reversal and the Subgingival Microbiota Study
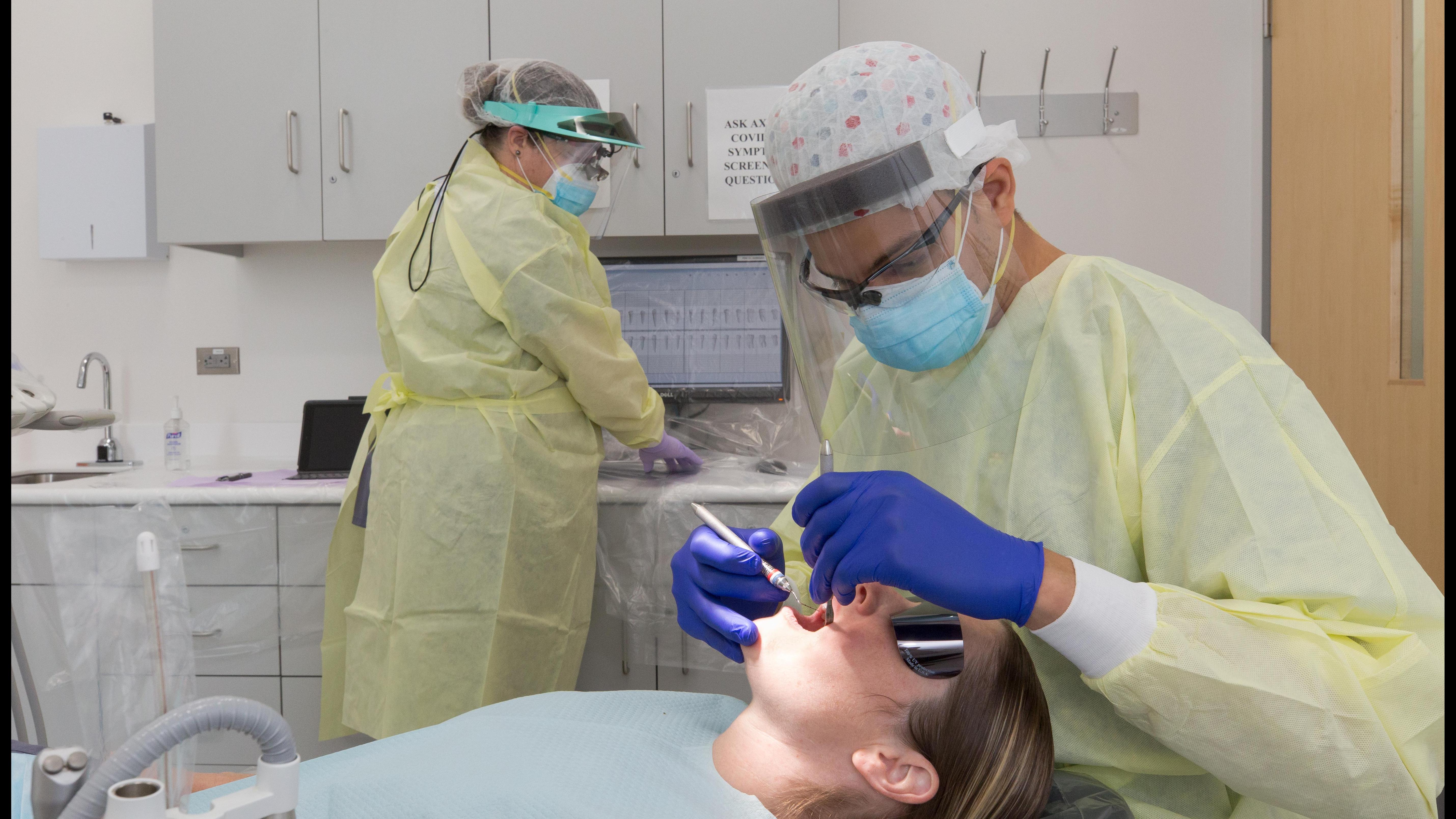
Brief description of study
The Penn Bariatric and Weight Loss Surgery Program at the University of Pennsylvania
is currently seeking individuals who are planning to undergo bariatric surgery and
willing to undergo an oral examination and provide oral samples at study visits before
and after the surgery. The purpose of this study to determine whether diabetes
alters the subgingival microbial composition and /or bacterial RNA expression by
comparing bacteria obtained from diabetic and nondiabetic individuals.
Detailed description of study
The study objective is to determine whether diabetes alters the oral microbial
composition and /or bacterial RNA expression by comparing bacteria obtained from
diabetic and nondiabetic individuals before and after bariatric surgery.
Eligible participants will be enrolled prior to their bariatric surgery and will attend
5 visits over the course of 18 months.
Participants who qualify for the study and attend all study visits will receive up
to $400. Additionally, after completion of the study participants will receive a
complimentary dental cleaning.
Eligibility of study
You may be eligible for this study if you meet the following criteria:
-
Conditions:
diabetes,bariatric surgery,weight loss
-
Age: Between 25 Years - 65 Years
-
Gender: All
- Subject Inclusion Criteria
- Men and women undergoing bariatric surgery who are 25-65 years old.
- BMI > 35 kg/m2.
- Diabetic subjects: HbA1c> 6.75% or fasting plasma glucose >126 mg/dl).
- Normoglycemic subjects: HbA1c<5.75% or fasting plasma glucose <100 mg/dl).
- Dental criteria: Minimum of 8 posterior teeth.
- Signed and dated informed consent form.
- Willing to comply with all study procedures and be available for the duration of the study.
- Subject Exclusion Criteria
- Subjects diagnosed with type 1 diabetes, maturity onset diabetes of the young (MODY), or latent autoimmune diabetes in adults (LADA).
- Women who are considering pregnancy or are currently breastfeeding.
- Individuals with a history of chronic inflammatory or autoimmune diseases or taking medications that affect immune function or affect body weight such as chronic systemic steroids.
- Currently smoke more than 10 cigarettes per day.
- Periodontal treatment within 3 months of bacterial sampling.
- Acute periodontal infection (abscess) within 1 month of bacterial sampling or abscess in teeth or adjacent teeth within 1 month of sampling.
- Treatment with antibiotics within one month of sample collection (except as part of the surgical regimen of prophylactic antibiotic immediately prior to surgery).
- A treatment regimen of steroids within 1 month of sample collection.
Updated on
09 Mar 2024.
Study ID: 825975
Find a site
,
You have contacted , on
Your message has been sent to the study team at ,
A copy of the message has been sent to your email
What happens next?
- You can expect the study team to contact you via email or phone in the next few days.
- Sign up as volunteer to help accelerate the development of new treatments and to get notified about similar trials.
You are contacting