18F-fluorodeoxyglucose (FDG) Positron Emission Tomography (PET/CT) for the evaluation of response to therapy in bone-dominant metastatic breast cancer
Brief description of study
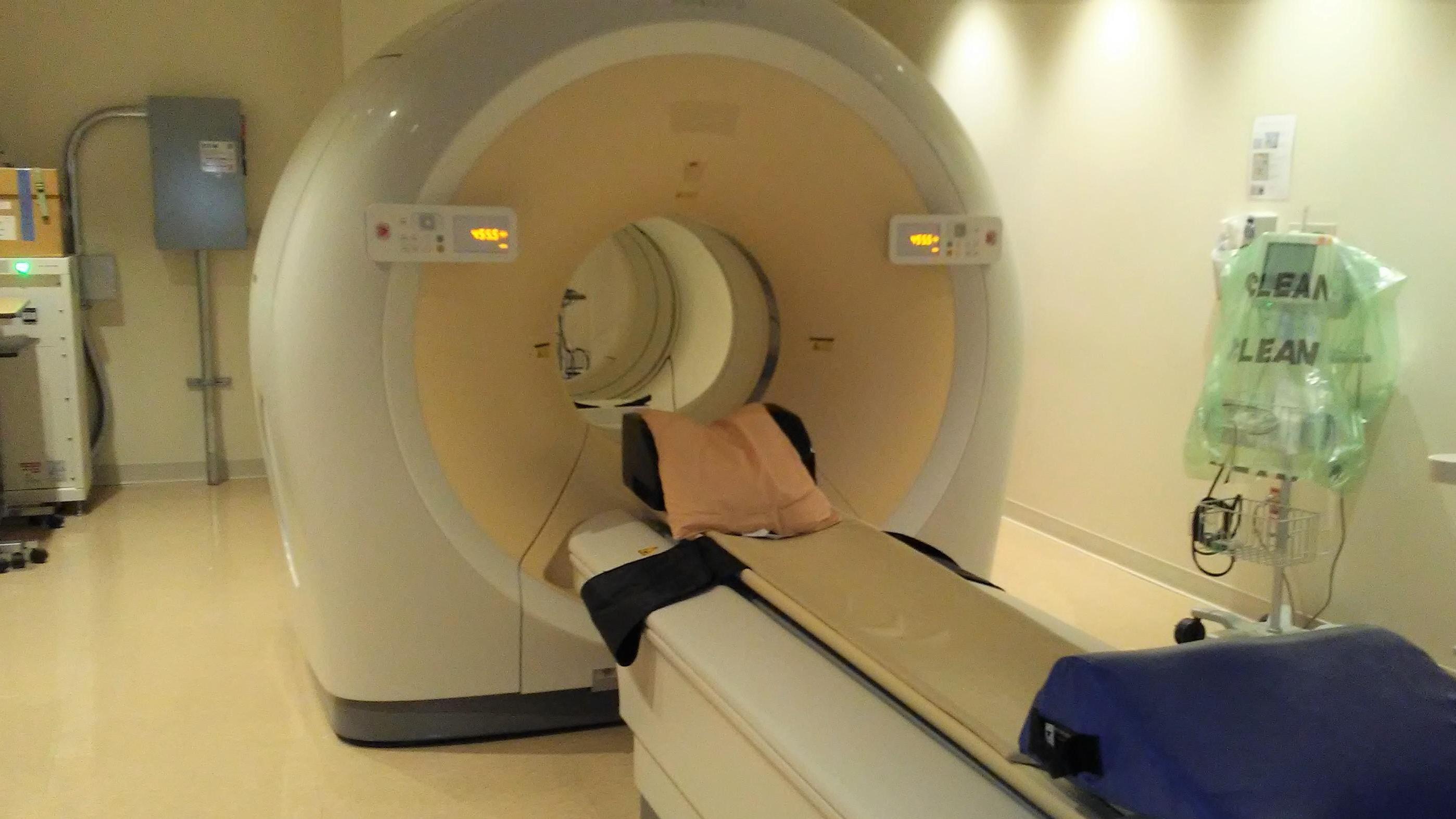
The purpose of this study is to learn more about breast cancer that has spread to the bones, called bone metastases. Measuring how bone metastases respond to treatment can be difficult with standard imaging, such as bone scans and MRI, and tumor markers alone. In this study, we will use an imaging method called Positron Emission Tomography (PET/CT) to visualize the response of bone metastases to treatment.
Detailed description of study
Up to 75 patients with metastatic breast cancer will take part in this study. All patients will have FDG PET/CT scans before starting new therapy, at one month and at 3 months after starting therapy (this scan may occur up to 4 months after treatment start at the discretion of your doctor). After completion of the 3 month scan active participation will be completed. After your active participation is completed we will continue to follow your medical records to collect information about your treatment for breast cancer.
Eligibility of study
You may be eligible for this study if you meet the following criteria:
-
Conditions:
cancer,breast cancer,,breast cancer r,breast cancer r,breast cancer r
-
Age: Between 18 Years - 90 Years
-
Gender: Female
Updated on
08 Jul 2023.
Study ID: 817940